How to properly charge lead-acid batteries
Modern technologies are developing by leaps and bounds, and with them the manufacturing technologies of lead-acid batteries are developing. In today's market, what kind of lead-acid batteries you will not find. Let's try to understand, characterize and classify them.
Operating modes
Let's dwell a little on the operating modes of batteries. So, it is customary to distinguish between the following modes of operation:
- Cyclic mode;
- Buffer mode;
- Mixed mode.
Cycling
A mode of operation in which the battery cycles from full discharge to full charge during use. This mode is considered "hard". This is because the life of a cycling battery is limited to a certain number of cycles. And the number of these cycles is not large, on average 300 - 600 cycles. In cyclic mode, such a battery will last from 6 to 12 months. An example of using a battery in a cyclic mode: children's electric cars.
Buffer mode
A mode of operation in which the battery is always connected to a power source during operation and only occasionally partially discharged. This mode is the most common and is considered "soft". Since during operation, the battery operating in the buffer mode is not completely discharged. The life of a battery operating in this mode is measured by time and averages from 1 to 5 years or more. An example of using a battery in buffer mode: uninterruptible power supplies.
Mixed Mode
A mode of operation in which the battery operates mainly in buffer mode during operation, and only occasionally in cyclic mode. This mode is a combination of buffer and loop modes in different proportions. The life of the battery operating in this mode depends on the very proportion of the mode. The more buffer mode, the longer the service life, and vice versa. Mixed mode battery example: car.
Battery types
It is customary to distinguish between the following types:
- LA (Lead Acid) - Lead Acid;
- SLA (Sealed Lead Acid) - sealed lead acid;
- VRLA (Valve Regulated Lead Acid) - sealed lead acid with internal pressure control valve.
Technological and design features
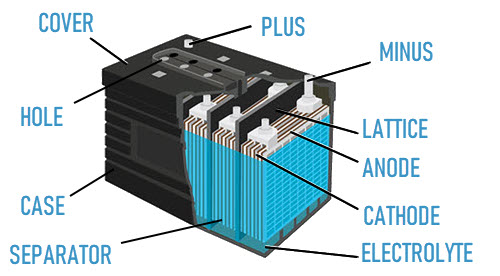
The design of all lead-acid batteries is similar and intuitive from the name. But still, let's dwell on it a little. A lead-acid battery consists of electrodes (plates, electrode grids) made of lead and an electrolyte, which is a solution of sulfuric acid (H2SO4), while the acid is dissolved in distilled water. Consider the structure of the battery in more detail.
The electrodes are mainly of a lattice structure coated with a special powder (active mass). In modern lead-acid batteries, pure lead is almost never found and additional metals are used in their manufacture. & nbsp; The plates, during manufacture, are equipped with various solid substances - active mass, for example: lead oxides, antimony, calcium. The coating of the electrode, the active mass, is usually called "spread". The plates are separated from each other by dividing bars. Dividing grids are called "separators". Summarize. The design of a modern lead-acid battery includes the following "elements":
- Grid plates (electrodes: Anode and Cathode);
- Active mass (plate spreading);
- Separators;
- Electrolyte
In the manufacture of "elements", different manufacturers use different materials and technologies. Hence the variety of types and types of modern batteries. According to technological and design features, modern lead-acid batteries are divided into manufacturing technologies:
- WET (Wet Cell Battery) - "wet" battery with "free" acid;
- EFB (Enhanced Flooded Battery) - reinforced "wet" battery;
- AFB (Advanced Flooded Battery) - advanced "wet" battery;
- ECM (Enhanced Cycling Mat) - modified lead-acid battery;
- GEL (Gelled Electrolyte) - gel electrolyte;
- AGM (Absorbent Glass Mat) - absorbent glass mat;
- AGM+ (Advanced Absorbent Glass Mat) - advanced absorbent glass mat;
- DRYFIT GEL- advanced gel technology;
- CARBON GEL - (carbon) battery.

WET (Wet Cell Battery) - "wet" battery with "free" acid.
Batteries of this type are the most common "classic" batteries with liquid electrolyte. The cycle life of WET batteries is: 250–500 cycles. The duty cycle (DOD) is: 50% depth of discharge. Standard charging voltage: 14.4-14.5V. An example of using a WET battery: a car battery. WET accumulators, in turn, are divided into:
- Serviced;
- Low maintenance (hybrid);
- Unattended.
Serviced Batteries. In serviced batteries, the electrodes are made of lead with the addition of antimony (PbSb). At the same time, the electrode grids absorb the electrolyte substance, so its concentration and quantity must be periodically monitored. This means that from time to time, distilled water should be added, while controlling the concentration of sulfuric acid. Concentration control is reduced to monitoring the overall density of the electrolyte every 3 - 6 months.
Low maintenance (hybrid) batteries. In hybrid batteries, the structure of the electrodes is different. Anode plates - are a combination of lead and low content antimony. Cathode plates - are a combination of lead and calcium. In the design of hybrid batteries, water consumption is reduced. Water is added rarely, mainly when recharging (boiling) or when operating in hot weather conditions.
Maintenance-free batteries. Maintenance-free batteries do not contain antimony. Anode plates - are a combination of lead, calcium, tin or silver. In the maintenance-free battery design, water consumption is further reduced. Water is not added at all. The status of a maintenance-free battery is monitored by the color of the built-in indicator (if any). The indicator has the following colors:
- Green - Battery is charged;
- Red - Low battery;
- White - Low electrolyte balance.

EFB (Enhanced Flooded Battery) - enhanced "wet" battery.
Batteries of this type are advanced economical lead-acid batteries with a liquid electrolyte, designed for use in cars with a Start / Stop system. The plates of these batteries are reinforced with layers of fiberglass to prevent shedding of the plates. These batteries are very resistant to vibration and are designed for use in "harsh" conditions. At the same time, EFB batteries are able to withstand 2 times more cyclic load and have a 16% longer duty cycle (500 - 1000 cycles). The duty cycle (DOD) is: 60% depth of discharge. Standard charging voltage: 14.4-14.5V. An example of using an EFB battery: a micro-hybrid vehicle battery.

AFB (Advanced Flooded Battery)- an advanced "wet" battery.
Batteries of this type are advanced economical lead-acid batteries with a liquid electrolyte, designed for use in cars with a Start / Stop system. The cathode plates of these batteries and the active mass are more resistant to various corrosive processes. The cathode plates are made from a unique alloy. AFB technology batteries have excess electrolyte. Separators have 2 layer partitions. At the same time, AFB batteries have an extended cycle life and more powerful starting capabilities. The cyclic resource of this type of battery is: 500 - 1000 cycles. The duty cycle (DOD) is: 60% depth of discharge. Standard charging voltage: 14.4-14.5V.

ECM (Enhanced Cycling Mat) - modified lead-acid battery.
Batteries of this type are advanced economical lead-acid batteries with a liquid electrolyte designed for use in cars with a Start / Stop system. The cathode plates of these batteries are made of foamed metal, and the active mass has a higher density. ECM technology batteries have an excess of electrolyte. At the same time, the ECM Batteries have extended cycle life and more powerful starting capabilities. The cyclic resource of this type of battery is: 500 - 1000 cycles. The duty cycle (DOD) is: 60% depth of discharge. Standard charging voltage: 14.4-14.5V.
Batteries made using EFB / AFB / ECM technology have similar characteristics and are intermediate between classic and AGM batteries.

GEL (Gelled Electrolyte) - gel electrolyte
The maintenance-free VRLA type gel batteries are advanced economical lead-acid batteries with silica gel as an electrolyte, designed for use in uninterruptible power systems, inverters, solar storage, etc. and are traction batteries. Silica gel allows you to increase the capacity of the battery, as it prevents evaporation. Gel batteries are VRLA type. Inside, gel batteries have a similar structure to liquid electrolyte batteries. Gel batteries do not contain antimony. Calcium is used in the active mass. The gelation of the electrolyte is achieved by adding a silicon compound. Gel batteries provide less electrolyte evaporation, increased cycle life (300 - 500 cycles) and increased resistance to shock, vibration and acid stratification. The duty cycle (DOD) is: 50% depth of discharge. Standard charging voltage: 14.2-14.3V. Gel batteries still have commercial applications, but are limited in use due to the rapid development of AGM technology.

AGM (Absorbent Glass Mat) - absorbent glass mat
Batteries of this type are maintenance-free VRLA type batteries designed for operation in uninterruptible power systems, inverters, solar storage, etc. and are traction batteries. Instead of gel, AGM batteries use absorbent fiberglass separators, which improves their efficiency compared to liquid and gel batteries. AGM batteries have lower internal resistance, which allows them to deliver more current. The cyclic resource of this type of battery is: 400 - 600 cycles. The duty cycle (DOD) is: 80% depth of discharge. Standard charging voltage: 14.7-14.8V. AGM battery charges 5 times faster.
Note: Like all VRLA batteries, AGM batteries are sensitive to improper charging.
 < br>
< br>
AGM+ (Advanced Absorbent Glass Mat) - advanced absorbent glass mat
AGM+ or Advanced AGM batteries are maintenance free VRLA type traction batteries. AGM+ batteries are similar in performance to traditional AGM batteries, but have a longer cycle life and lower internal resistance, making them suitable for micro-hybrid vehicles with a start/stop system. The cyclic resource of this type of batteries is: 800 - 1200 cycles. The duty cycle (DOD) is: 80% depth of discharge. Standard charging voltage: 15.2-15.3V.

DRYFIT GEL - advanced gel technology
Batteries of this type are maintenance-free VRLA batteries with a jelly-like electrolyte, designed for use in uninterruptible power systems, alarms, medical equipment, inverters, solar storage, river and sea transport, measuring instruments, etc. The anode plate is made on a tubular technologies. The gelatinization of the electrolyte is achieved by adding a special thickener to it. A microporous material is used as a separator. DRYFIT batteries do not contain antimony. Antimony has been replaced by calcium. Batteries with DRYFIT technology are resistant to deep discharges, charge very quickly, and last longer than classic free acid batteries. DRYFIT batteries have a longer cycle life (1200 - 2000 cycles) compared to AGM batteries. Duty cycle (DOD) is: 80% depth of discharge.

CARBON GEL- (carbon) batteries
Batteries of this type are maintenance-free VRLA batteries with a jelly-like electrolyte and are traction batteries. Carbon batteries are universal, with increased cycling, and, accordingly, their scope is energy storage, electric vehicles, wheelchairs, golf carts, warehouse and other equipment. But in uninterruptible power supplies, their use is not recommended. The electrodes in such batteries are made using nano-tubular technology. Carbon has been added to the plates. The electrolyte used is a colloidal electrolyte with the addition of silicon dioxide. Carbon is also added to the active mass of the plates, which significantly reduces the sulfation process. The cyclic resource of this type of batteries is: 3000 - 4800 cycles. The duty cycle (DOD) is: 30% - 70% depth of discharge. Standard charging voltage: 14.4-15.0V. Currently released carbon batteries: I and II generation.
CARBON I generation: cannot be discharged with high currents, but can be fully discharged.
CARBON II generation: can be discharged with high currents, but cannot be fully discharged. Discharge limit: 30%.
Note: The number of cycles of carbon batteries corresponds to the number of cycles of lithium batteries.
Note: Carbon batteries are now on the market capable of providing high starting currents, which means that batteries of this type are becoming suitable for use in cars.
As you can see, from the above, there are quite a lot of manufacturing technologies for modern batteries. Each "technology" has its own requirements for the charging procedure and requirements for charging parameters. We touched on some of the charging parameters above, let's touch on the charging procedure a bit. The whole procedure for charging a modern battery is usually divided into stages. Each type of battery has its own set of procedures and the number of stages. For example, VRLA batteries do not have a "Desulfate" stage, and also do not have a "Recovery" stage. The most common charging steps are listed below. Consider these steps using the same 12V batteries as an example.
Charging steps
Stage 1 (Desulfatation)- Desulfation
Definition of sulfated batteries LA, SLA types. Desulfation of VRLA batteries is not necessary due to the high quality of the metal. Charging occurs by applying a pulsed voltage, allowing sulfates to be removed from the surface of the lattice plates, thereby restoring the capacity of the battery. Pulse voltage: 15.6V
Stage 2 (Soft Start) - Soft Start
At this stage, the battery is tested for charge susceptibility. The test takes place at a voltage of 12.6V with a current of 0.1C
Stage 3 (Bulk)- Main charge
At this stage, the LA, SLA, VRLA batteries are charged up to 80% of the rated capacity with the maximum direct current (CC). Charging current 0.1C The charging voltage is constantly increasing, reaching the maximum allowable value in cyclic mode. The maximum allowable voltage value in cyclic mode - each type of battery has its own. It is marked on the battery. For example, for LA, SLA types it averages 14.4V, and for AGM batteries it averages 14.7V
Stage 4 (Absorption)- Absorption
At this stage, LA, SLA, VRLA batteries are charged up to 100% of the rated capacity with the maximum constant voltage (CV) in cyclic mode. The charging current in this mode is constantly decreasing.
Step 5 (Analyse) - Diagnosis
At this stage, the battery is tested to see if it holds a charge. The essence of testing is to measure the battery voltage for 3 minutes. The battery voltage should not drop below 12V (depending on the battery voltage). If the battery fails the test at stage 5, such a battery is considered unusable.
Stage 6 (Recond)- Restoration
This step is only suitable for LA, SLA battery types. For VRLA batteries, this step is contraindicated. During this stage, the battery charging voltage is increased to 15.8V with a current of no more than 1.5A in order to cause controlled gas evolution (boiling). Gas evolution promotes electrolyte mixing, thereby restoring electrolyte separation and increasing battery capacity. Separation of the electrolyte (stratification) can occur either due to natural causes or after maintenance (topping up with distilled water).
Stage 7 (Float)- Saturation Mode or Buffer Mode
At this stage, the voltage of the LA, SLA, VRLA batteries is maintained at the maximum allowable value in buffer mode, by supplying a constant voltage. The value of the maximum support voltage averages 13.6V
Stage 8 (Pulse)- Preventive exercise
At this stage, the charge of LA, SLA, VRLA batteries is maintained at the level of 95% - 100%. The charger in this mode constantly monitors the battery charge and, if necessary, recharges it, thereby keeping the battery fully charged and ready for use.
Summary
We can summarize: a modern battery is almost impossible to charge with a classic old-style charger with manual adjustment of the charging current. It is necessary to use modern pulse chargers with full automation of charging processes. In such chargers, the stages and parameters are already prescribed by the manufacturer. And consequently, the probability of damaging the battery is reduced to a minimum.
Occurring abbreviation
DOD - Depth of Discharge - depth of discharge of the battery;
С – nominal battery capacity at 10-hour discharge and temperature 25°C;
1 reviews / Write a review